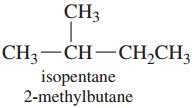PRINCIPLES
OF NOMENCLATURE
In early days of organic chemistry,
each new compound was given an individual name. Such name was based on the source,
some property, or some other trivial reason. The common names are like
nicknames.
For Ex - Formic acid (HCOOH)
was named as it was obtained by distillation of red ants (Latin, formica =
ants). Barbiturates after the name of a
woman Barbara.
IUPAC
SYSTEM OF NOMENCLATURE
v With the rapid
growth of organic chemistry, the number of compounds increased day by day (now about
6 million).
v It became impossible
to give common names to such a large number of compounds.
v In 1957, the International
Union of Pure and Applied Chemistry evolved a scheme for giving systematic
names to organic compounds on the basis of structure. This is known as the IUPAC
System.
v This system has
set rules for naming organic molecules from their structures.
v The systematic
name of a compound derived from its structural formula by applying IUPAC rule is
referred to as its IUPAC Name.
v One organic compound
can have only one IUPAC name.
v Knowing the IUPAC name
of a compound, we can write its
structural formula.
v However, common
names of the first few members of a homologous series have been retained in the
IUPAC system.
v Naming organic
compounds is an essential skill. You must be able to give correct name for a Structure
or derive a correct structure from a name.
How
to Name Organic Compounds
The
naming of organic compounds are in five parts. You should name molecules
starting backwards from this list. For example, determine functional group
first, then saturation, then the parent chain, then the substituents, and then
the stereoisomer characteristics:
Stereoisomer Characteristics Substituents Parent
Chain Bond Types Functional Groups
1.
Stereoisomer Characteristics
a.
Cis (Z) /Trans (E)
1.
Cis – identical groups on same side
2.
Trans – identical groups on opposite sides
b.
(R)/(S)
To
determine whether R or S, find the direction of the priority groups numbered
1-3.
Lowest priority group (#4) must be in back when doing this.
1.
(R)- priority #1-3 are clockwise
2.
(S)- priority #1-3 are anti- clockwise
c.
(+)/(-)
i.
Identifies rotation under plane-polarized light
ii.
To determine whether molecule is (+) or (-), need to do laboratory tests where
the molecule is put in plane-polarized light
1.
(+) – rotates clockwise
2.
(-) – rotates anti-clockwise
2.
Substituents
a.
After determining the functional group and parent chain, everything else that
is left are considered substituents.
b.
Carbon chain- Name using
following names based on number of carbons. Numbers correlate with amount of
carbons in chain:
Number
of carbons in substituent chain Name
1
Methyl 2 Ethyl 3 Propyl 4 Butyl 5
Pentyl
6
Hexyl 7 Heptyl 8 Octyl 9 Nonyl 10
Decyl
c.
Branched carbon substituents (not connected in
straight line like usual):
isopropyl: tert-butyl:
d.
Halide functional groups- take the halogen
name (ex. Chlorine) and remove the “-ine”
and
add an “-o” in place for the resulting name (ex. Chloro)
e.
Include any other functional groups that
were not part of the parent chain. (Ex. –oxy- for
ethers)
f.
Rings- If parent chain
is in a ring, add cyclo- to the beginning of parent name.
g.
Give number to indicate location of substituent on parent chain.
(ex.
3-methylpentane indicates a methyl group on the 3rd carbon of the parent chain
of pentane)
3.
Parent Chain
a.
Name chain with following names in accordance to number of carbons in parent
chain.
Number
of carbons in substituent chain Name
1
Meth- 2 Eth- 3 Prop- 4 But- 5
Pent-
6
Hex- 7 Hept- 8 Oct- 9 Non- 10
Dec.
To determine parent chain:
i.
Try to look for longest chain of carbon in molecule. This is the parent chain.
ii.
If there is a functional group, double bond and/or triple bond, include the functional
group in the parent chain. Then try to include the double bond, then the triple
bond. This may require choosing a shorter parent chain.
4.
Bond Types
a.
–ane-; alkanes; single bonds only
in structure
b.
–ene-; alkenes; double bonds
exist in structure
c.
–yn-; alkynes; triple bonds
exist in structure
d.
If more than one double or triple bond use prefixes:
2.
Di- 3.
Tri- 4.
Tetra- 5.
Penta- 6.
Hexa-.
If
both double and triple bonds exist, list double bond then triple bond. (ex.
2,3,5-triene- 4,5-diyne)
5.
Functional Groups
a.
Written as a suffix at the end of compounds name.
b.
-3-ol – Give number to indicate the carbon that the functional group is
attached to
c.
If there is more than one functional group, put the one higher on the hierarchy
in the suffix and the others in the substituents.
d.
–e; If no functional group
attached, end the name with an “e”. (ex) pentane)
e.
Some functional groups are carboxylic acid, aldehyde, ketone, amines, alcohols etc.
I.
NOMENCLATURE OF ALKANES
v Alkanes are
hydrocarbons that contain only single bonds. (The compounds that contain only
carbon and hydrogen).
v The first four
members of the series are known by their common names: Methane, Ethane,
Propane, and Butane.
v The names of
larger alkanes are derived from the Greek prefixes that indicate the number of
carbon atoms in the molecule. Thus pentane has 5 carbons, hexane has
6, and so on.
v General Formula
for alkane is CnH2n + 2
v In the common
system all isomeric alkanes have the same parent name.
For
example, two isomers in C4H10 alkanes are known as
butanes. The names of various isomers are distinguished by prefixes. The prefix
indicates the type of branching present in the molecule.
(1) Meaning of the
Prefix n- Prefix
n- is used for those alkanes in which all carbons are in a continuous
chain. The prefix n- stands for normal or straight-chain.
CH3-CH2-CH2-CH2-CH3 n-Pentane
(2)
Meaning of the Prefix Iso- Prefix iso- is used for those alkanes
which have a methyl group (CH3-) attached to the second last carbon
atom of the continuous chain.
(3) Meaning of the Prefix Neo- Prefix neo- is
used for those alkanes which have two methyl
groups
attached to the second last carbon atom of the continuous chain.
Classification of
Carbon Atoms
The
structural formulas of alkanes contain four types of carbons:
(1) Primary Carbon
(1°). A carbon atom attached to one other
(or no other) carbon atom is called primary carbon.
(2) Secondary
Carbon (2°).
A carbon atom attached to two other carbon atoms is called secondary carbon.
(3) Tertiary
Carbon (3°).
A carbon atom attached to three other carbon atoms is called tertiary carbon.
(4) Quaternary
Carbon (4°).
A carbon atom attached to four other carbon atoms is called quaternary carbon.
Alkyl Groups
An
alkyl group is formed by removing one hydrogen atom from an alkane.
The symbol R- is often used to
represent an alkyl group. The grouping R- (e.g.CH3CHr) is a compound and must
be bonded to another atom or group of atoms. Alkyl groups are named dropping -ane
from the name of the corresponding alkane, and adding the ending-yl.
Non
alkyl Groups
A number of non alkyl groups are
used in naming organic compounds. For example
-Cl = Chloro -Br = Bromo -I
= Iodo
-F
= Fluoro -NO2 = Nitro -NO
= Nitroso
-NH2 = Amino -OH
= Hydroxy
Straight Chain Alkanes
|
# Carbon
|
Name
|
Molecular
Formula |
Structural
Formula |
|
1
|
Methane
|
CH4
|
CH4
|
|
2
|
Ethane
|
C2H6
|
CH3CH3
|
|
3
|
Propane
|
C3H8
|
CH3CH2CH3
|
|
4
|
Butane
|
C4H10
|
CH3CH2CH2CH3
|
|
5
|
Pentane
|
C5H12
|
CH3CH2CH2CH2CH3
|
|
6
|
Hexane
|
C6H14
|
CH3(CH2)4CH3
|
|
7
|
Heptane
|
C7H16
|
CH3(CH2)5CH3
|
|
8
|
Octane
|
C8H18
|
CH3(CH2)6CH3
|
|
9
|
Nonane
|
C9H20
|
CH3(CH2)7CH3
|
|
10
|
Decane
|
C10H22
|
CH3(CH2)8CH3
|
A) IUPAC Rules for
Naming Alkanes
The IUPAC system is the same for all
classes of organic compounds. The IUPAC rules for naming alkanes are given
below.
Rule1. Select the
longest continuous carbon chain. Remember that this chain does not have to be that
portion of the molecule that is written horizontally.
Rule2. Name the longest chain.
The longest carbon chain is chosen as the basis for the name.
Rule3. Number the
longest chain. The carbon atoms in the longest chain are numbered. The numbering
is started from that end which will give numbers having the lowest value to carbons
carrying substituents.
Rule4. Identify the
substituent. Name the substituent. Indicate its position by the number of the carbon
atom to which it is attached.
Rule5. Prefix the
position number and name of the substituent onto the parent name. The whole name
is written as one-word. Notice that the number and name of the substituent are
separated by a hyphen.
Rule6 Identify the substituents by names and
position numbers. When the same substituent present two or more times in the
molecule, prefixes di, tri-, tetra-, penta-, etc. are used. Position of
each substituent is indicated by a separate number. These position number separated
by commas, are put just before the name of the substituent, with the hyphen before
and after the numbers when necessary.
Rule7. When two or more
different substituents are present, their names are arranged in alphabet order
and added to the name of the parent alkane, again as one word
B.
Alkanes: Branched-Chains
The straight simple-chain alkanes
have simple names and can be easily memorized, but the branched-chain alkanes
require a set of simple rules derived by the IUPAC.
Basic
Rules:
1.
Identify the parent chain, longest carbon chain in the molecule.
If there is more than one carbon chain of
equal length, then identify the chain that is more substituted.
2.
Number the carbon atoms in the longest carbon chain, from the end that gives
the substituents as low a number as possible.
3.
Name all the substituents, groups that are attached to the parent chain.
The substituents are named by their
respective prefix and end with -yl.
Example: CH3- methyl, CH3CH2-ethyl
There are some common
"special" names for branched alkane substituents which should be memorized.
A few of them are:
4.
Assign numbers to substituents to identify where the attachment of the
substituent is to the parent chain.
You must still assign a number to each substituent along with the
prefixes di-, tri-tetra- etc., even
if there are the same substituents in the molecule.
Example:
1,2-dimethyl, 1-methyl
5.
Add the prefixes, suffixes together, remembering to alphabetize substituents in
the complete name.
i.
The prefixes cyclo-, iso-, and neo- are considered part of the group
name so they are alphabetized.
ii.
Ignore the prefixes di-, tri-¸tetra-,
tert-, sec-, etc., when alphabetizing.
iii.
Use commas between numbers and dashes between numbers and words. Example: 1, 3-dimethyl
iv.
If you are required to describe the isomer, you may also need identify whether
the molecule is R or S, cis or trans, E/Z, etc.