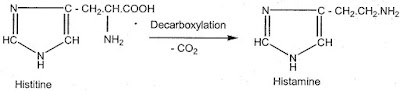
Histaminie is a beta imidazole ethylamine derivative which is present in essentially all mammalian tissues. In the living organism histamine is synthesized from the naturally occurring a - amino acid, histidine by the loss of a carboxyl group through bacterial or enzymatic decarboxylation.
 Histamine is produced naturally by human system and released in response to tissue damage. In human beings, histamine cause immediate allergic and inflammatory response cause gastric acid release and function as a CNS neuro transmitter.
Histamine is produced naturally by human system and released in response to tissue damage. In human beings, histamine cause immediate allergic and inflammatory response cause gastric acid release and function as a CNS neuro transmitter.Systemically, histamine contracts smooth muscle of lungs and gastro intestinal system and cause vasodilatation, low B.P and increase heart rate. It causes symptoms such as itching, sneezing, watery eye and running nose.
Histamine receptors
The physiological effects of histamine are mediated by specific cell-surface receptors. These receptors are divided into three types.
1) H1- receptors
It is found in smooth muscles of intestine, bronchi, blood vessels, adrenal medulla, endothelial cell and lymphocytes. Histamine H1 receptors are G-protein linked receptors. It is sequence of 491 amino acids residue. The third and fifth membrane domain is responsible for binding histamine.
H1 - receptors mediate smooth muscle contraction, increased vascular permeability, pruritus, prostaglandin, decreased artrio ventricular conduction time accompanied by tachycardia and activation of vagal reflexes.
2) H2 - receptors:
They are located on the cell membrane of acid secreting cells of gastric mucosa and mediate the gastric acid secretary actions of histamine. The physiological effects of H2 - receptor ligands are mediated by a stimulatory G- protein coupled receptor which activates adenylate cyclase / cyclic AMP intra cellular second messenger. The TM3 aspartate and aspartate and threonine residue in TM5 is responsible for binding histamine.
3) H3 - receptors :
It is presynoptic receptor that influences the release of histamine and other neuro transmitters from neurons.
Anti Histamines
Anti histamines are drugs which inhibit the action of histamine by competitively blocking the histamine receptors.
They are located on the cell membrane of acid secreting cells of gastric mucosa and mediate the gastric acid secretary actions of histamine. The physiological effects of H2 - receptor ligands are mediated by a stimulatory G- protein coupled receptor which activates adenylate cyclase / cyclic AMP intra cellular second messenger. The TM3 aspartate and aspartate and threonine residue in TM5 is responsible for binding histamine.
3) H3 - receptors :
It is presynoptic receptor that influences the release of histamine and other neuro transmitters from neurons.
Anti Histamines
Anti histamines are drugs which inhibit the action of histamine by competitively blocking the histamine receptors.
A) HISTAMINE H1 RECEPTOR ANTAGONIST:
Classification :
I. First generation anti histamines :
1) Amino alkyl ethers: Diphen hydramine HCl, Dimenhydrinate, Bromodiphenhydramine HCl, Doxylamine succinate, Carbinoxamine maleate, Clemastine fumerate, Diphenyl pyraline HCl.
2) Ethylene diamines: Tripelennamine HCl, Pyrilamine maleate, Metha pyrilene HCl, Thonzylamine HCl, Antazoline PO4.
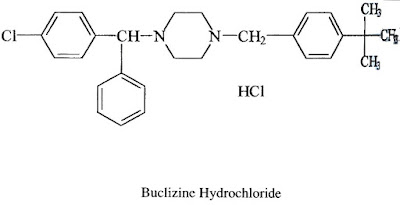
3) Piperazine derivatives : Cyclizine HCl, Chlorcyclizine HCl, Meclizine HCl, Buclizine HCl.
4) Propylamine derivatives (Mono amino propyl derivatives): Pheniramine maleate,
Chlor Pheniramine maleate, Triprolidine HCl, Phenindamine tartarate, Pyrrobutamine PO4, Dimethindene maleate, Dexchlorpheniramine maleate, Brompheniramine maleate, Dex brompheniramine maleate.
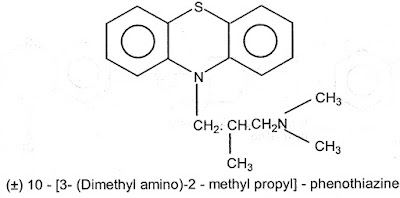
5) Phenothiazine derivatives : Promethazine HCl, Trimeprazine tartarate, Methdilazine.
6) Dibenzocyloheptene derivatives : Cyproheptadine HCl, Azatadine maleate.
II. Second generation H1 antagonist: Terfenadine, Astemizole, Loratadine, Cetirizine, Acrivastine.
III. Inhibition of Histamine release (Mast cell stabilizers) : Cromolyn sodium,
Nedocromil sodium.
Nedocromil sodium.
B) HISTAMINE H2 RECEPTOR ANTAGONIST :
Cimetidine, Famotidine, Ranitidine, Nizatidine.
Cimetidine, Famotidine, Ranitidine, Nizatidine.
C) OTHER ANTI ULCER AGENTS:
Omeprazole, Lansoprazole, Pantoprazole, Rabeprazole.
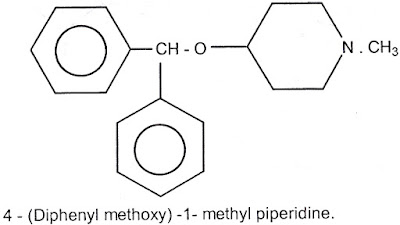
1) Amino alkyl ethers
The General formula is
(Ar)2 – CH – O – CH2CH2 N (R)2
Omeprazole, Lansoprazole, Pantoprazole, Rabeprazole.
1) Amino alkyl ethers
The General formula is
(Ar)2 – CH – O – CH2CH2 N (R)2
Doxylamine
Doxylamine Synthesis
Clemastine
Clemastine Synthesis
Diphenyl pyraline
- The aromatic group may be phenyl or substituted phenyl or heterocyclic for good antihistaminic activity.
- P – substituted aromatic groups have good activity but O – substitution in aryl groups loss the activity.
- Removal of a1 – methyl group and insertion of chlorine in to Para position of the phenyl ring in Doxylamine enhanced activity.
- Substitution of 2 – thionyl for 2 – pyridyl group decreased the activities.
- Compounds with an asymmetric carbon atom, the Dextro isomer is more active.
- If double bond is introduced between a , b carbon atoms of the propyl chain drowsiness will be developed.
- One of the aryl group is 2 – pyridinyl system is more significant anti histaminic activity.
- Substitution of P – methoxy (Pyrilamine), chloro (Chlor pyramine) or bromo (Brom tripelenamine) group enhances the activity.
- The two Tertiary nitrogens are separated by two carbon chain for good activity. Extension or branching of this chain decrease activity.
- The Tertiary nitrogen may be a part of heterocyclic ring (antazoline) also has good activity.
3) Piperazine derivatives - P-Substitution of any one aryl group by chlorine enhances the anti histaminic activity.
- The both nitrogen atoms of Piperazine are aliphatic and have basicities for good activities.
- The R- group may be methyl or aralkyl for good activity.
- One of the aryl group may be 2 – Pyridinyl group is more significant antihistaminic activity.
- Introduction of chlorine in P- position of benzyl group has 20 times more potent than un substituted compounds.
- These drugs have an asymmetric carbon atom, the Dextro isomers exhibiting the greater potency.
- In the unsaturated derivatives, the trans isomers are more active.
- The tertiary nitrogen may be a part of heterocyclic ring (Triprolidine) has greatest activity.
- The side chain of phenothiazines contain two or three carbon atoms, branched alkyl chain between ring system and terminal nitrogen atom gives good anti histaminic activity.
- The phenothiazines with a 3 carbon bridge between nitrogen atoms are more potent in vitro.
- The 30 nitrogen of side chain may be a part of heterocyclic ring also shows good activity.
Ethylene Diamines
The General formula is
(Ar)2 N - CH2CH2 -N (CH3)2SAR for ethylene diamines :
(Ar)2 N - CH2CH2 -N (CH3)2
 SAR for Piperazine Derivatives :
SAR for Piperazine Derivatives :Propylamine derivatives
The General formula is
(Ar)2 CH CH2CH2 -N (R)2
PhenindamineSAR for Propylamines :
V. Phenothiazine derivatives :
Trimeprazine
SAR for Phenothiazines
6.Di benzo cycloheptanes
III. Inhibition of Histamine release (Mast cell stabilizers) MECHANISM OF ACTIONFor histamine H1 antagonist :
- H1 antagonist mainly competitively inhibit the action of histamine on tissues containing H1 receptors.
- The drugs have the pharmacological actions, opposite to that of histamines and also prevent the access of histamine to its receptors by competitive antagonism.
- Some antihistamines also antagonize serotonin and bradykinin which are released along with histamine during anaphylaxis reaction.
- Stimulation of H1 receptors leads to an increase of the intracellular calcium concentration and hydrolysis of phosphatidylino – sitol -4,5-bis phosphate to inositol triphosphate (IP3) and 1,2 diacyl glycerol (DAG). So these histamine induced production of IP3 and DAG is antagonized by H1 receptor antagonist.
- The increased intra cellular level of IP3 and DAG by histamine is the mobilization of intra cellular calcium.
- Elevation of intracellular calcium level is associated with various biochemical consequences including the activation of PLC and phospholipase A2, which liberates arachidonic acid from cell membrane, leading to production of powerful mediator like prostacyline and thromboxene A2.
- In intestine smooth muscles histamine activate the ion channels permeable to Na+ and K+ leading to depolarization and muscle contraction.
- So the H1 antagonist competitively inhibits the histamine H1 receptor and prevent the above actions.
B) HISTAMINE H2 RECEPTOR ANTAGONIST :
SAR for H2 Antagonist :
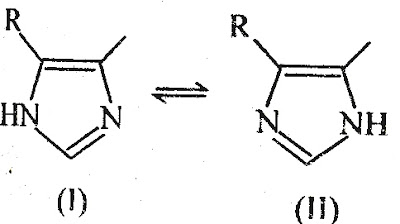
- Imidazole ring exist in two tautomeric forms. In these form – I to be necessary for maximal H2 antagonist activity.
- When R is substituted with methyl group, the activity is potentiated.
- The other heterocyclics like furan, thiazole are enhance the potency and selectivity of H2 receptor antagonism.
- The ring and terminal nitrogen should be separated by four carbon atom for optimum activity. The shorter chain decrease the activity.
- The side chain should contain an electron with drawing substituents and an Isosteric thioether (- S-) link in place of methylene group (- CH2) leads to more active compound.
- The terminal nitrogen should be polar, non basic substituents for maximal activity.
- Though a positively charged group binds more tightly to the receptor, it leads to an agonist activity rather than an antagonist activity.
C) OTHER ANTI ULCER AGENTS:
Mechanism of action For Histamine H2 antagonists :
- H2 antagonists mainly antagonize the action of histamine at its H2 receptors which is responsible for acid secretion and peptic ulcer disease.
- Mucus secreted by the gastric mucous cells combined with surface epithelial bicarbonate secretion contributes to a barrier that prevents gastric acid and pepsin from damaging the gastric mucosa.
- The acid secretary unit of gastric mucosa is the parietal cell which contain a hydrogen ion pump, H3O+ - K+ - ATP ase system that secretes H3O+ is exchange for the uptake of K+ ion.
- Secretion of acid by gastric parietal cells is regulated by the actions of various mediators at receptors including histamine agonism of H2 receptors.
- Since H2 – receptor antagonists are potent inhibitors of all stimulants of gastric acid secretion, histamine may be considered as a single common final mediator of acid secretion on the parietal cells.
- The H2 antagonists simple inhibit the direct actions of histamine on acid secretion.
H2 antagonists also protect mucosal barriers, proton pump inhibitors, prostaglandins.
