ANXIOLYTICS, SEDATIVES AND HYPNOTICS
These drugs are under the category of CNS depressants.
Sedatives are drugs which decreases activity and excitement of the patients and calm the anxiety by producing mild depression of CNS with out causing drowsiness or sleep.
These drugs are under the category of CNS depressants.
Sedatives are drugs which decreases activity and excitement of the patients and calm the anxiety by producing mild depression of CNS with out causing drowsiness or sleep.
Hypnotics are drugs which produce drowsiness and induce sleep resembling to natural sleep by depressing CNS.
Anxiolytics are also called as minor tranquillizers which are used in treatment of psychotic disorder by depressing CNS. They are used to treat abnormalities of mental functions.
The various sedatives and hypnotic agents are also employed as :
i) Antianxiety agents in emotional strain and chronic tension ii) Anticonvulsant
iii) Muscle relaxants iv) General anaesthetic v) Potentiation of analgesic drugs
vi) Adjuvants to anesthesia vii) In hyper tension
Classification :
I. Barbiturates:-
1. Long acting barbiturates (More than 8hours) – Barbitone, Barbital sodium,
Phorobarbital, Mephobarbital
2. Intermediate acting barbiturates (4 - 8hours) – Allo barbital, hexobarbitone,
pento hexital sodium
3. Short acting barbiturates (less than 4 hours) – Seco barbitone, hexobarbitone,
Pento barbitone, cyclo barbitone
4. Ultra short acting barbiturates ( I-V anaesthetics) – Thiopental sodium.
Phorobarbital, Mephobarbital
2. Intermediate acting barbiturates (4 - 8hours) – Allo barbital, hexobarbitone,
pento hexital sodium
3. Short acting barbiturates (less than 4 hours) – Seco barbitone, hexobarbitone,
Pento barbitone, cyclo barbitone
4. Ultra short acting barbiturates ( I-V anaesthetics) – Thiopental sodium.
Metho hexital sodium
II. Benzodiazepines -
Diazepam, Chlordiazepoxide, oxazepam, chlorazepate dipotassium, prazepam,
Lorazepam, Halazepam, Flurazepam, Alprazolam, Triazolam
III. Amides and Imides –
Glutethimide, methyprylon, methaqualone
IV. Aldehydes and their derivatives –
Chloral hydrate, Paraldehyde, Triclofos sodium
V. Alcohol and their carbamate derivatives –
Ethinamate, Meprobamate
VI. Miscellaneous –
Antihistamines, KBr, Morphine, Pethidine
I.Barbiturates
I.Barbiturates
Barbiturates are cyclic diurides which are the derivative of barbituric acid( 2,4,6 tri oxo hexahydro pyrimidine). As such barbituric acid has no CNS depressant activities. But substitution at 5th position by alkyl or aryl groups confers sedative and Hypnotic activity.
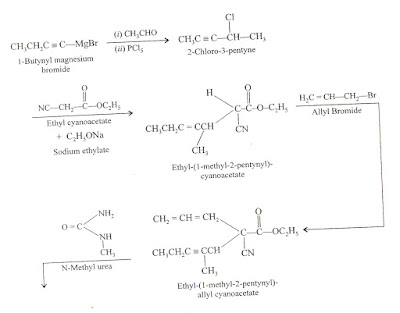
barbituric acid or barbiturates are prepared from malonic acid or its esters.
barbituric acid or barbiturates are prepared from malonic acid or its esters.
 1.Barbital (Barbitone sodium)
1.Barbital (Barbitone sodium)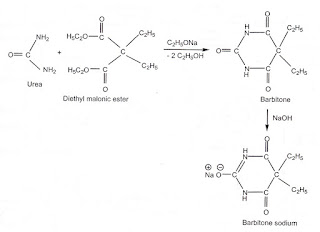
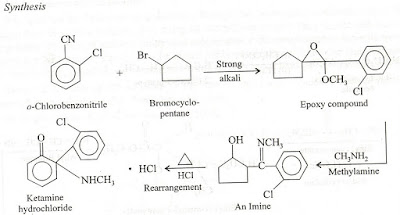
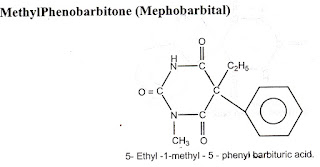
2.Pheno barbitone
Structure-activity relationship of barbiturates (SAR):
- Barbituric acid itself does not possess any sedative and hypnotic activity. The sedative
or hypnotic activity is produced when the two active hydrogen atoms at position 5,5
have appropriate substituents by alkyl or aryl groups. - The total number of carbon atoms present in two groups at 5th position must not be less
than 4 and more than 10 for their optiomal activity. - Only one of the substituents may be a closed chain for good activity
(Example : Hexobarbital, Cyclobarbital, Phenobarbital) - The branched chain isomer exhibits greater activity and shorter duration. The drug
having greater branching is more potent (Example : Pento barbital, amobarbital). - Double bonds are present in alkyl substituents groups produce compounds more
readily to tissue oxidation. Hence they are short acting (Example : Seco barbital) . - Stereo isomers have appropriately same potencies.
- Aromatic or alicyclic substituted analogues are more potent than the corresponding
aliphatic analogues having same number of carbon atoms. - Short chain at carbon 5 resists oxidation and hence long acting .Long chains are readily
oxidized and thus produce short acting (Example : Barbital, Pentobarbital, Seco barbital) - Introduction of halogen atom is to 5 – alkyl substituents increases potency
- Introduction of polar groups (OH, NH2, COOH, SO3H, CO, RNH) is to 5 – alkyl or
aryl substituents decrease lipid solubility and Potency. - Alkylation at 1 or 3 position enhances onset and reduce duration of action
(Example : Hexobarbital). - Replacement of oxygen by sulphur at 2 – carbon (thio barbiturates) shortens on set and duration of action due to increased lipid solubility. But more sulphur at C4SC6 decrease the activity (Example: Thio pental)
Mechanism of action
- Barbiturates either have a Gam amino butyric acid (GABA) like action or enhance the effect of GABA, an inhibitory transmitter.
- The effects of barbiturates on synaptic transmission are caused by an alteration of post synaptic sensitivity of the neurons to excitatory and inhibitory transmitters.
- When GABA receptors are activated, chloride channels are open and chloride enters the cell, hyper polarizes it and produces decreased excitation.
- Barbiturates bind to picrotoxin site of GABA receptor and decrease chloride ion flux and produce an increased chloride ion concentration.
They may interfere with the passage of impulses from centers in hypothalamus to the cortex.
Benzo diazepines:
Bendiazepines and their derivatives are mainly used for sedative, hypnotic, tranquillizer, muscle relaxant and anti convulsant.
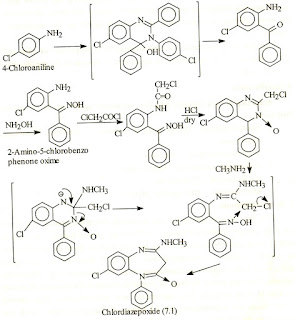
1. Diazepam
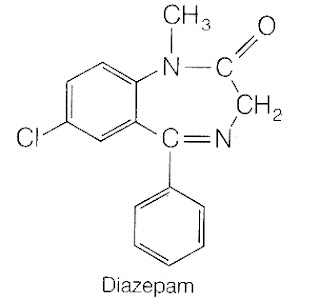 Synthesis of Diazepam
Synthesis of Diazepam 2.Chlor Diazepoxide
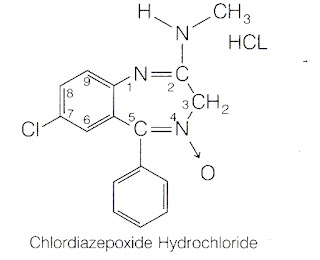
Synthesis of Chlor Diazepoxide
3. Oxazepam

3. Oxazepam
 4. Chlorazepate Dipotassium
4. Chlorazepate Dipotassium 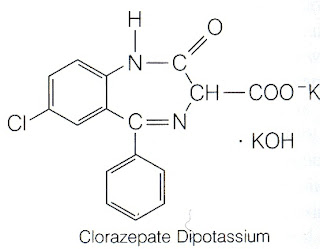 5. Lorazepam
5. Lorazepam
6.Halazepam
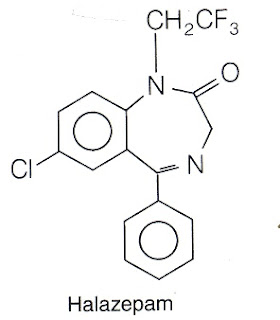
7.Flurazepam
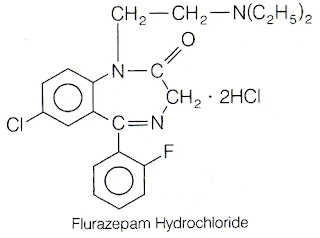
8. Alprazolam
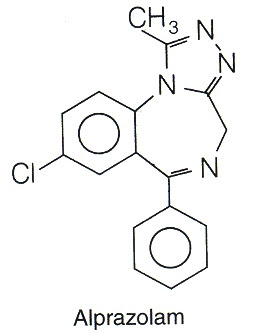
Structure-Activity Relationship Of Benzo Diazepines
- All Benzodiazepines are used as CNS depressant are usually substituted with a 5-aryl or 5- cyclo hexenyl groups .
- The substituents present in position 1 to 3 does not affect the activity.
- The N(4) usually substituted with a low electron density group.
- The electron with drawing group present in the position 7 is required for hypnotic activity. Position 6,8 & 9 should not be substituted.
- A phenyl group at 5th position promotes the activity. An electron withdrawing group in ortho or di ortho position increase the activity. (Eg. Lorazepam, Flurazepam)
- If 4,5 double bond is saturated or shift to 3,4th position decrease the activity.
- Alkyl substitution at 3rd position decrease the activity.
Mechanism of action
- The mid brain reticular activating system which is responsible for the maintenance of a wakefulness is depressed by benzodiazepines.
- The anti anxiety activity of benzodiazepines may be attributed to the depressant action of these drugs on the mechanism which evoke anxiety and aggression.
- Like GABA, the benzodiazepines cause either pre synoptic or post synoptic inhibition in poly synaptic neuronal pathways in CNS, affecting the various turnover of various neurotransmitters in the brain, therefore interfere the transmission process.
Trichlofos sodium